You are here: Home  Technical Articles
Technical Articles  Food,Flavours & Fragrances
Food,Flavours & Fragrances  HPLC Analysis of Glucosinolates in Vegetable Extracts
HPLC Analysis of Glucosinolates in Vegetable Extracts
 Technical Articles
Technical Articles  Food,Flavours & Fragrances
Food,Flavours & Fragrances  HPLC Analysis of Glucosinolates in Vegetable Extracts
HPLC Analysis of Glucosinolates in Vegetable Extractswithout Ion pairing using an Ultra Aqueous C18 Column
Glucosinolates are a naturall occuring set of compounds found in a variety of edible plants, most notably in broccoli, radish and cabbage. Agriculturally, the degradation compounds of glucosinolates have been shown to act as natural pesticides and fungicides. This breakdown occurs in the soil. These toxic compounds then further degrade into harmless compounds. Research on glucosinolates is continuing in hopes of bringing a more environmentall friendly approach to pest control.
Nutritionally, human consumption of these compounds is associated with a significantly reduced risk for a variety of malingnant cancers along the alimentary canal. They also have been shown to suppress existing tumor growth. Glucosinolates are precursors to isothiocyanates such as sulforaphane (4-methylsulfinybutyl isothiocyanate), which regulates mammalian enzymes of xenobiotic metabolism.
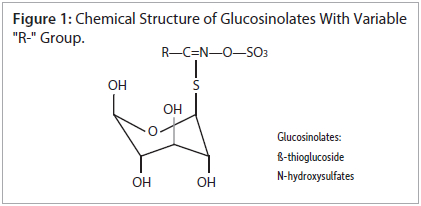
Phenethyl glucosinolate (gluconasturtiin) is one of the glucosinolates widely found in cruciferous vegetables. It is one of the least polar glucosinolates, making it relatively easy to retain by reversed-phase, high-performance liquid chromatography (HPLC). However, there are a number of glucosinolates with hydrophilic (R-" groups, such as 3-methylsulfinylpropyl glucosinolate, that are very difficult to retain by conventional reversed-phase HPLC. Additionally, the "R-" group of glucosinolates can vary greatly, resulting in a large number of glucosinolates with widely differing polarities (figure 1). Thus, many analysts resort to reversed-phase ion-pairing methods to analyze glucosinolates. The addition of ion-pairing reagents is less convenient, and makes the analyses inherently less reproducible. Ion-pairing reagents is less convenient, and makes the analyses inherently less reproducible. Ion-pairing reagents also make gradient elution very impractical, due to long equilibration times.
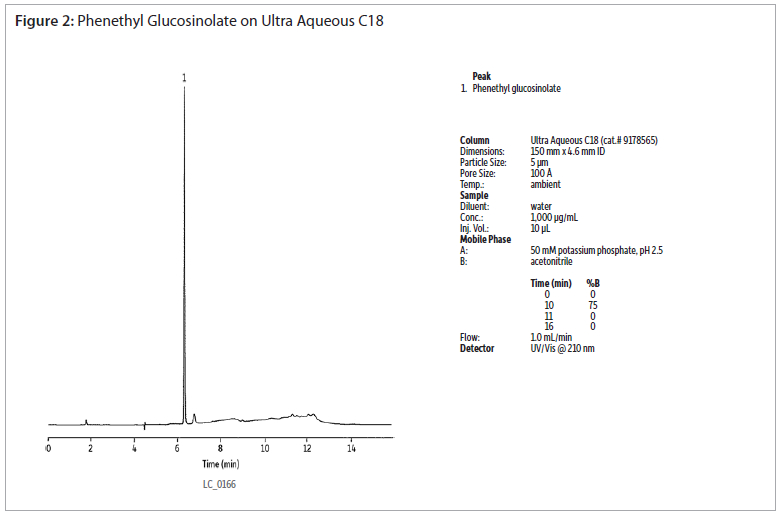
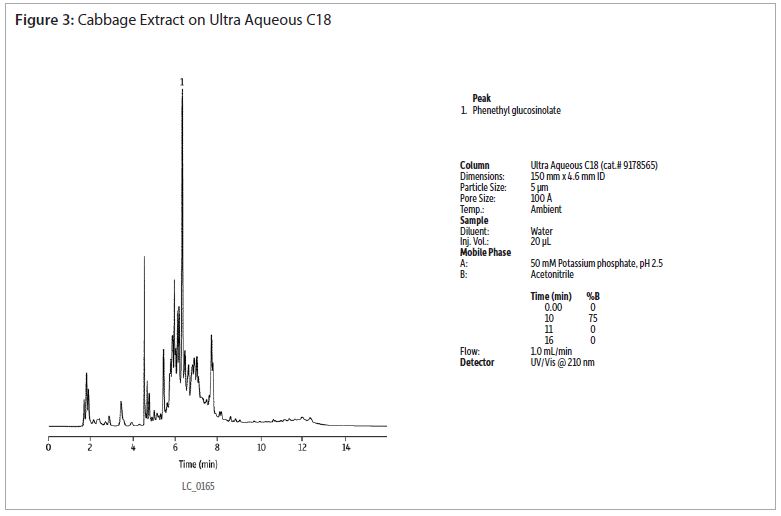
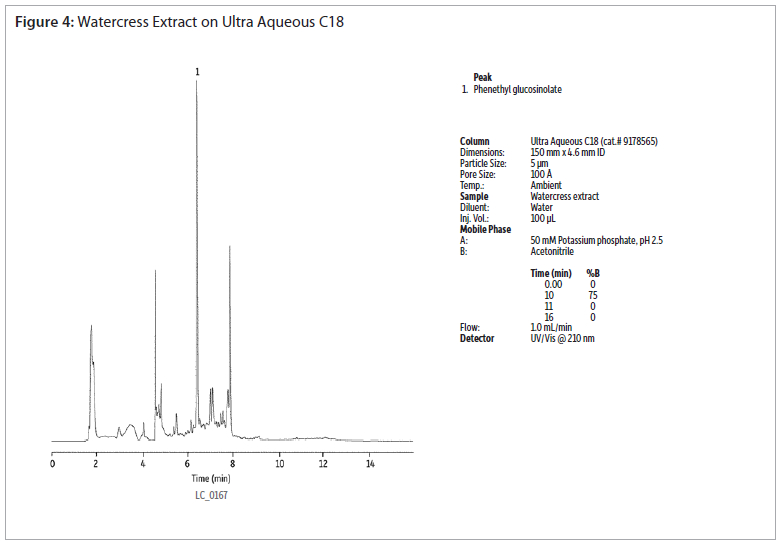
The analysis of a phenethyl glucosinolate standard using an Ultra Aqueous C18 column shows good peak shapre without the use of ion-pairing reagents (figure 2). Extracts of cabbage and watercress were analysed using the same conditions (figures 3 and 4). Gradient elution from 0 to 75% acetonitrile was used to retain and elute analytes having a wide range of polarities. The Ultra Aqueous C18 column allows the use of simple reversed-phase conditions for the analyses of glucosinolates, saving time as compated to reversed-phase ion-pairing methods.